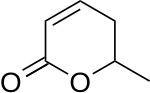
Parasorbic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name (6S)-5,6-dihydro-6-methyl-2H-pyran-2-one | |
| Other names 2-methyl-2,3-dihydropyran-6-one, 2-Hexen-5-olide, 5-hydroxy-2-Hexenoic acid δ-lactone, parasorbic acid, sorbic oil, γ-Hexenolactone, (+)-(6S)-Parasorbic acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| DrugBank |
|
| KEGG |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C6H8O2 |
| Molar mass | 112.128 |
| Appearance | colorless liquid |
| Density | 1.0 g/mL (estimated) |
| Boiling point | 227 °C (441 °F; 500 K) estimated |
Solubility in water | 50 g/L |
| Solubility | estimated |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) | -360.03 kJ·mol−1 |
| Hazards | |
| GHS labelling: | |
Pictograms |  |
| Warning | |
| H315, H319, H335 | |
| NFPA 704 (fire diamond) |  2 2 0 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Chemical compound
Parasorbic acid is the cyclic lactone of sorbic acid. Thermal treatment or hydrolysis converts the lactone to sorbic acid.[1]
Toxicity
Parasorbic acid is toxic and causes indigestion and nausea, however cooking and exposure to moisture convert it to the benign food preservative sorbic acid.[2]
See also
References
- ^ A. S. Naidu, ed. (2000). Natural food antimicrobial systems. p. 637. ISBN 0-8493-2047-X.
- ^ Mason PL, Gaunt IF, Hardy J, Kiss IS, Butterworth KR, Gangolli SD (1976). "Long-term toxicity of parasorbic acid in rats". Food Cosmet Toxicol. 14 (5): 387–394. doi:10.1016/S0015-6264(76)80174-5. PMID 1010506.











