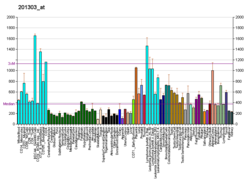
EIF4A3
| EIF4A3 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | EIF4A3, DDX48, MUK34, NMP265, NUK34, RCPS, eIF4AIII, eukaryotic translation initiation factor 4A3, Fal1, eIF4A-III, eIF-4A-III | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 608546; MGI: 1923731; HomoloGene: 5602; GeneCards: EIF4A3; OMA:EIF4A3 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Eukaryotic initiation factor 4A-III is a protein that in humans is encoded by the EIF4A3 gene.[5][6][7]
Function
This gene encodes a member of the DEAD box protein family. DEAD box proteins, characterized by the conserved motif Asp-Glu-Ala-Asp (DEAD), are putative RNA helicases. They are implicated in a number of cellular processes involving alteration of RNA secondary structure, such as translation initiation, nuclear and mitochondrial splicing, and ribosome and spliceosome assembly. Based on their distribution patterns, some members of this family are believed to be involved in embryogenesis, spermatogenesis, and cellular growth and division. The protein encoded by this gene is a nuclear matrix protein. Its amino acid sequence is highly similar to the amino acid sequences of the translation initiation factors eIF4A-I and eIF4A-II, two other members of the DEAD box protein family.[7]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000141543 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000025580 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Holzmann K, Gerner C, Poltl A, Schafer R, Obrist P, Ensinger C, et al. (Feb 2000). "A human common nuclear matrix protein homologous to eukaryotic translation initiation factor 4A". Biochem Biophys Res Commun. 267 (1): 339–44. doi:10.1006/bbrc.1999.1973. PMID 10623621.
- ^ Chan CC, Dostie J, Diem MD, Feng W, Mann M, Rappsilber J, et al. (Jan 2004). "eIF4A3 is a novel component of the exon junction complex". RNA. 10 (2): 200–9. doi:10.1261/rna.5230104. PMC 1370532. PMID 14730019.
- ^ a b "Entrez Gene: EIF4A3 eukaryotic translation initiation factor 4A, isoform 3".
Further reading
- Nagase T, Miyajima N, Tanaka A, Sazuka T, Seki N, Sato S, et al. (1995). "Prediction of the coding sequences of unidentified human genes. III. The coding sequences of 40 new genes (KIAA0081-KIAA0120) deduced by analysis of cDNA clones from human cell line KG-1". DNA Res. 2 (1): 37–43. doi:10.1093/dnares/2.1.37. PMID 7788527.
- Li Q, Imataka H, Morino S, Rogers Jr GW, Richter-Cook NJ, Merrick WC, et al. (1999). "Eukaryotic Translation Initiation Factor 4AIII (eIF4AIII) Is Functionally Distinct from eIF4AI and eIF4AII". Mol. Cell. Biol. 19 (11): 7336–46. doi:10.1128/mcb.19.11.7336. PMC 84727. PMID 10523622.
- Jurica MS, Licklider LJ, Gygi SR, Grigorieff N, Moore MJ (2002). "Purification and characterization of native spliceosomes suitable for three-dimensional structural analysis". RNA. 8 (4): 426–39. doi:10.1017/S1355838202021088. PMC 1370266. PMID 11991638.
- Lee S, Baek M, Yang H, Bang YJ, Kim WH, Ha JH, et al. (2002). "Identification of genes differentially expressed between gastric cancers and normal gastric mucosa with cDNA microarrays". Cancer Lett. 184 (2): 197–206. doi:10.1016/S0304-3835(02)00197-0. PMID 12127692.
- Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Palacios IM, Gatfield D, St Johnston D, Izaurralde E (2004). "An eIF4AIII-containing complex required for mRNA localization and nonsense-mediated mRNA decay". Nature. 427 (6976): 753–7. Bibcode:2004Natur.427..753P. doi:10.1038/nature02351. PMID 14973490. S2CID 4400243.
- Ferraiuolo MA, Lee CS, Ler LW, Hsu JL, Costa-Mattioli M, Luo MJ, et al. (2004). "A nuclear translation-like factor eIF4AIII is recruited to the mRNA during splicing and functions in nonsense-mediated decay". Proc. Natl. Acad. Sci. U.S.A. 101 (12): 4118–23. Bibcode:2004PNAS..101.4118F. doi:10.1073/pnas.0400933101. PMC 384704. PMID 15024115.
- Shibuya T, Tange TØ, Sonenberg N, Moore MJ (2004). "eIF4AIII binds spliced mRNA in the exon junction complex and is essential for nonsense-mediated decay". Nat. Struct. Mol. Biol. 11 (4): 346–51. doi:10.1038/nsmb750. PMID 15034551. S2CID 30171314.
- Jin J, Smith FD, Stark C, Wells CD, Fawcett JP, Kulkarni S, et al. (2004). "Proteomic, functional, and domain-based analysis of in vivo 14-3-3 binding proteins involved in cytoskeletal regulation and cellular organization". Curr. Biol. 14 (16): 1436–50. Bibcode:2004CBio...14.1436J. doi:10.1016/j.cub.2004.07.051. PMID 15324660. S2CID 2371325.
- Gerhard DS, Wagner L, Feingold EA, Shenmen CM, Grouse LH, Schuler G, et al. (2004). "The Status, Quality, and Expansion of the NIH Full-Length cDNA Project: The Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Andersen JS, Lam YW, Leung AK, Ong SE, Lyon CE, Lamond AI, et al. (2005). "Nucleolar proteome dynamics". Nature. 433 (7021): 77–83. Bibcode:2005Natur.433...77A. doi:10.1038/nature03207. PMID 15635413. S2CID 4344740.
- Xia Q, Kong XT, Zhang GA, Hou XJ, Qiang H, Zhong RQ (2005). "Proteomics-based identification of DEAD-box protein 48 as a novel autoantigen, a prospective serum marker for pancreatic cancer". Biochem. Biophys. Res. Commun. 330 (2): 526–32. doi:10.1016/j.bbrc.2005.02.181. PMID 15796914.
- Ballut L, Marchadier B, Baguet A, Tomasetto C, Séraphin B, Le Hir H (2005). "The exon junction core complex is locked onto RNA by inhibition of eIF4AIII ATPase activity". Nat. Struct. Mol. Biol. 12 (10): 861–9. doi:10.1038/nsmb990. PMID 16170325. S2CID 1359792.
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, et al. (2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. Bibcode:2005Natur.437.1173R. doi:10.1038/nature04209. PMID 16189514. S2CID 4427026.
- Gehring NH, Kunz JB, Neu-Yilik G, Breit S, Viegas MH, Hentze MW, et al. (2005). "Exon-junction complex components specify distinct routes of nonsense-mediated mRNA decay with differential cofactor requirements". Mol. Cell. 20 (1): 65–75. doi:10.1016/j.molcel.2005.08.012. PMID 16209946.
- Andersen CB, Ballut L, Johansen JS, Chamieh H, Nielsen KH, Oliveira CL, et al. (2006). "Structure of the exon junction core complex with a trapped DEAD-box ATPase bound to RNA". Science. 313 (5795): 1968–72. Bibcode:2006Sci...313.1968A. doi:10.1126/science.1131981. PMID 16931718. S2CID 26409491.
- You KT, Li LS, Kim NG, Kang HJ, Koh KH, Chwae YJ, et al. (2007). "Selective Translational Repression of Truncated Proteins from Frameshift Mutation-Derived mRNAs in Tumors". PLOS Biol. 5 (5): e109. doi:10.1371/journal.pbio.0050109. PMC 1854916. PMID 17456004.
- Zhang Z, Krainer AR (2007). "Splicing remodels messenger ribonucleoprotein architecture via eIF4A3-dependent and -independent recruitment of exon junction complex components". Proc. Natl. Acad. Sci. U.S.A. 104 (28): 11574–9. Bibcode:2007PNAS..10411574Z. doi:10.1073/pnas.0704946104. PMC 1913901. PMID 17606899.
- v
- t
- e
-
 2hxy: Crystal structure of human apo-eIF4AIII
2hxy: Crystal structure of human apo-eIF4AIII -
 2hyi: Structure of the human exon junction complex with a trapped DEAD-box helicase bound to RNA
2hyi: Structure of the human exon junction complex with a trapped DEAD-box helicase bound to RNA -
 2j0q: THE CRYSTAL STRUCTURE OF THE EXON JUNCTION COMPLEX AT 3.2 A RESOLUTION
2j0q: THE CRYSTAL STRUCTURE OF THE EXON JUNCTION COMPLEX AT 3.2 A RESOLUTION -
 2j0s: THE CRYSTAL STRUCTURE OF THE EXON JUNCTION COMPLEX AT 2.2 A RESOLUTION
2j0s: THE CRYSTAL STRUCTURE OF THE EXON JUNCTION COMPLEX AT 2.2 A RESOLUTION -
 2j0u: THE CRYSTAL STRUCTURE OF EIF4AIII-BARENTSZ COMPLEX AT 3.0 A RESOLUTION
2j0u: THE CRYSTAL STRUCTURE OF EIF4AIII-BARENTSZ COMPLEX AT 3.0 A RESOLUTION